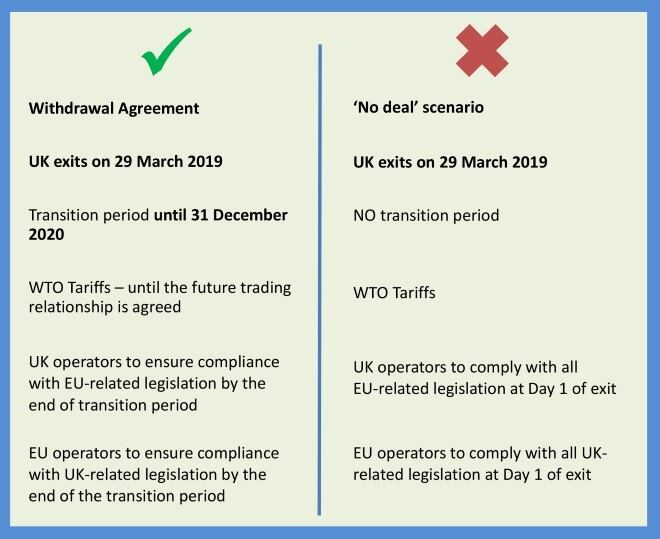
The document, “The Impact of a ‘No deal’ Brexit on the Cosmetics Industry”, outlines for cosmetic companies (members and non-members) how a ‘no deal’ scenario would impact on their business. It is available in full on the CTPA website.
Two relevant UK Government technical notices
The UK Government has published a series of technical notices to provide guidance to businesses on how to prepare if the UK leaves the EU with no deal.
The CTPA new factsheet suggests that the two papers most relevant to the cosmetics industry are:
- Appointing nominated persons to your business if there’s no Brexit deal (available here)
- Regulating chemicals (REACH) if there’s no Brexit deal (available here)
More information specifically on cosmetics is expected to be published later in 2018.
Appointing nominated persons to your business if there’s no Brexit deal
This document specifically mentions the Cosmetics Regulation, and clarifies that: “For cosmetics, responsible persons based in an EU country will no longer be recognised by the UK after March 2019 (Regulation (EC) N° 1223/2009 is the main EU legislation covering cosmetics and covers the role of the responsible person).
Businesses wishing to place cosmetics on the UK market will need to appoint a UK-based responsible person. This is due to specific legal duties assigned to the responsible person and their importance on ensuring the safety of products placed on the market.”
Regulating chemicals (REACH) if there’s no Brexit deal
This document states that a UK REACH legislation will replace the EU REACH legislation for the UK market.
This will establish a UK regulatory framework and domestic capacity to deliver the functions currently performed by ECHA.
The Health & Safety Executive (HSE) will be the competent authority; there will be a specialist capacity to evaluate the impact of chemicals on health and the environment.
The new UK legislation will maintain existing standards of protection of human health and the environment and will established a new UK REACH IT system similar to the EU REACH IT to enable registrations for REACH UK.
In case of a ‘no deal’ scenario, UK companies that registered under EU REACH will have to transfer the registrations to an EU-based Only Representative to continue to access the EU market.
This will require action before the UK leaves the EU. With regard to the UK REACH, the document establishes a step approach for compliance taking into account different supply chain scenarios.




