Promotional Features
Using microencapsulation to unlock resveratrol’s commercial potential in skincare
Dr Shaher Duchi, PhD; Dr Danny Goldstein, PhD
Resveratrol’s properties make it a promising skincare ingredient but formulation challenges have prevented the realization of this potential. Tagra Biotechnologies is seeking to clear these barriers using microencapsulation technology.
Resveratrol contains a compound that inhibits an oxidizing agent, has anti-inflammatory and antimicrobial properties and exerts stronger antioxidant effects than vitamin E and C.1-4 Studies show it can stop skin damage and improve skin firmness, elasticity and density.5-7
Yet, resveratrol has struggled to gain traction in skincare, with consultancy Future Market Insights noting that formulation issues are “denting consumer confidence” and “posing challenges vis-à-vis its adoption.”8
Resveratrol is not water-soluble so has to be dissolved using other means, such as organic solvents and oils. Additionally, resveratrol is an unstable ingredient. Under the effect of light and high pH, trans-resveratrol is transitions to the less active cis-form, limiting the duration of efficacy of topical formulations.9,10
Microencapsulation, a technology that protects, stabilizes and controls the release of active ingredients, could overcome these shortcomings.11-13 Tagra has a track record of using microencapsulation to enhance the stability of a range of active ingredients and to manage their release profiles.14,15
To assess if the technology can address the limitations of resveratrol, Tagra prepared and tested microcapsules of the ingredient, named CelluCap Resveratrol.16.17
Results
Microcapsule characterization
Trans-resveratrol content was quantified using HPLC and the entrapment efficacy determined (Table 1). The calculated yield of the microcapsule preparation was 85% to 90%.
Parameters | ||
Yield (%) | Content (%w/w) | Encapsulation efficiency (%) |
86.2 ± 1.5 | 14.2 ± 1.0 | 88.8 ± 1.7 |
Table 1. Mean yield (%), content (% w/w) and entrapment efficacy (%) trans-resveratrol in CelluCap Resveratrol microcapsules (mean ± SD, n= 4)
Particle Size
Particle size analysis results are presented in Table 2. The mean diameter of the CelluCap Resveratrol microcapsules was 37.64 ± 4.04 µm (mean ± SD). The microcapsule population was homogenous in size.
Mean size | % Particle population | ||
| 90 | 50 | 10 |
37.64 ± 4.04 | 50.01 ± 4.93 | 34.25 ± 4.75 | 3.5 ± 1.2 |
Table 2. Mean microcapsules size and size distribution by volume (diameter; mean ± SD, n=4)
Chemical stability
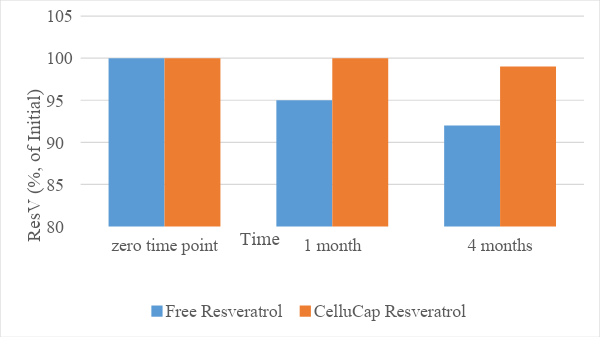
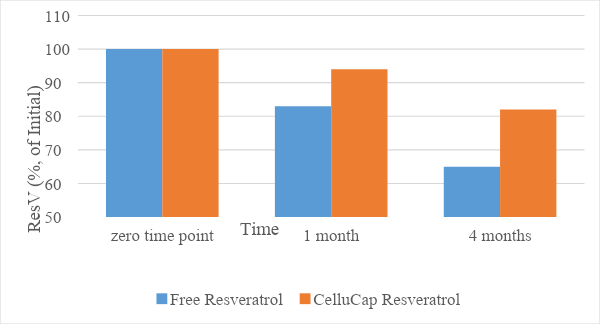
The stability of the encapsulated resveratrol compared to its free form was evaluated following incorporation of each compound into a topical oil-in-water (o/w) formulation. The relative content of trans-resveratrol (% of initial) in each sample was evaluated following storage at 25 and 40°C for 4 months. The results (Figures 1 and 2) indicate that the mean relative content of resveratrol over time remained significantly higher in encapsulated formulations compared to formulations containing free resveratrol. Thus, CelluCap Resveratrol microcapsules protect resveratrol from oxidation and degradation.
Figure 1. Stability of CelluCap Resveratrol microcapsules compared to free resveratrol incorporated in topical o/w formulation after 1 and 4 months storage at 25°C. Data are presented as mean % of initial content (n = 3).
Figure 2. Stability of CelluCap Resveratrol microcapsules compared to free resveratrol incorporated in topical o/w formulation after 1 and 4 months storage at 40 °C. Data are presented as mean % of initial content (n = 3).
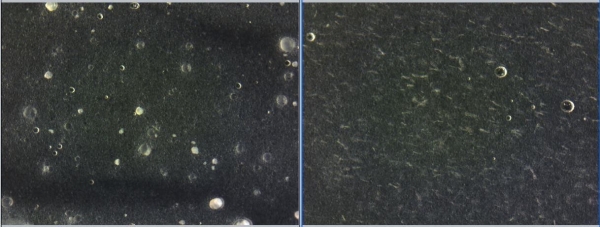
The formulations were tested under light microscopy to detect recrystallization. Free form resveratrol recrystallized over time at 25 and 40 C storage conditions. In contrast, CelluCap Resveratrol showed no observed leakage or recrystallization during the study (Figure 3).
Organoleptic evaluation of the formulations shows discoloration in the formulation that contains free resveratrol even at a storage condition of 25 C, compared to no discoloration of the formulation with CelluCap Resveratrol (Figure 4).
Figure 3. Light microscopy micrograph of formulations containing 4.25% CelluCap Resveratrol (left) or 0.6% free resveratrol (right) following 4 months storage at 25 C.
Figure 4. Organoleptic evaluation of formulations containing 4.25% CelluCap Resveratrol (left) or 0.6% free resveratrol (right) following 4 months storage at 25 C.
Photo stability
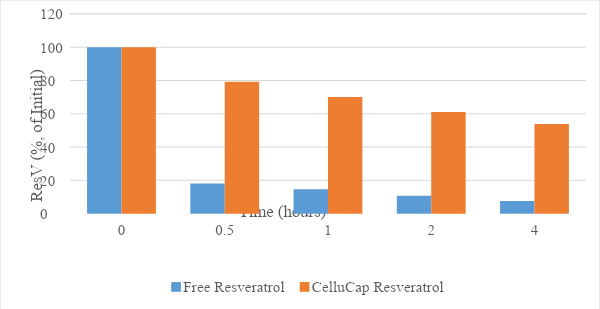
Photo stability results (Figure 5) indicate that the mean relative content of resveratrol over time remained significantly higher in formulations containing encapsulated resveratrol compared to formulations containing free resveratrol, showing encapsulation has a significant impact on the level of trans-resveratrol degradation.
After 4 hours of sun light irradiation, 53.8% of the initial trans-resveratrol was present in CelluCap Resveratrol, compared to 7.3% in the free form. Thus, CelluCap Resveratrol microcapsules successfully protect resveratrol from degradation following UV exposure.
Figure 5. Photo-stability of CelluCap Resveratrol microcapsules compared to free resveratrol incorporated in topical o/w formulation after 0.5, 1, 2 and 4 hours sun light exposure. Data are presented as mean % of initial content (n = 3).
UV-induced trans-cis isomerization was greatly reduced in CelluCap Resveratrol. More than 50% of the original concentration of trans isomer was preserved. Capsulation inhibits UV-induced trans-cis isomerization.
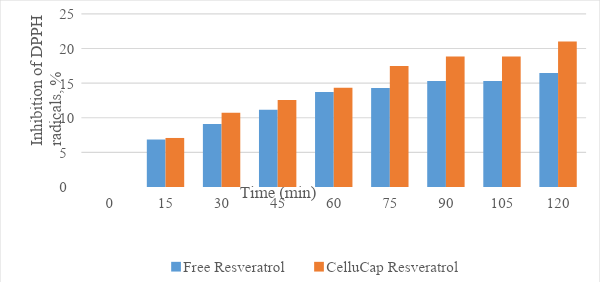
Determination of the antioxidant activity by DPPH test
Trans-resveratrol encapsulation significantly increased the formulation’s ability to "scavenge" DPPH radicals (Figure 6).
Figure 6. Antioxidant activity of CelluCap Resveratrol microcapsules compared to free resveratrol. Final trans-resveratrol in both tested samples is 0.1mg/mL.
Clinical Results
Methodology:
The study was designed as an open, intra-individual study; each subject is her own control comparing.
The tested parameters were evaluated before and after twice daily treatment for 28 days by using 3D Primos Lite System and Cutometer.
The tested parameters were: Rz: average relief, Rt: maximum relief amplitude and R0: cutaneous firming (firmness).
The tested product is Tagra Lotion which contains 5% CelluCap Resveratrol as the only active ingredient (equivalent to 0.7% un-encapsulated resveratrol).
The objectives of the study were to evaluate:
1. The smoothing/anti-wrinkle effect of the studied product by measurements using 3D
Primos Lite System.
2. The effect on the skin biomechanical properties by measurements using Cutometer.
3. Subjectively it’s cosmetic acceptability, its efficacy and future use by analysis of the subjects’ answers to a subjective evaluation questionnaire.
Results and conclusions:
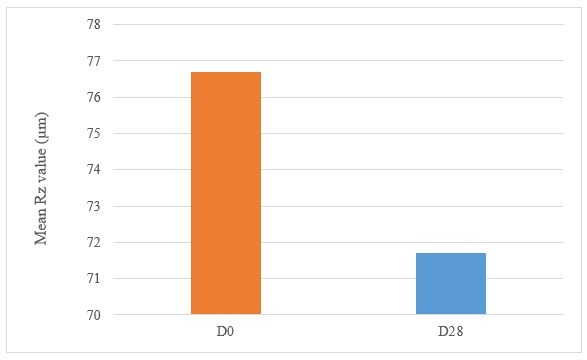
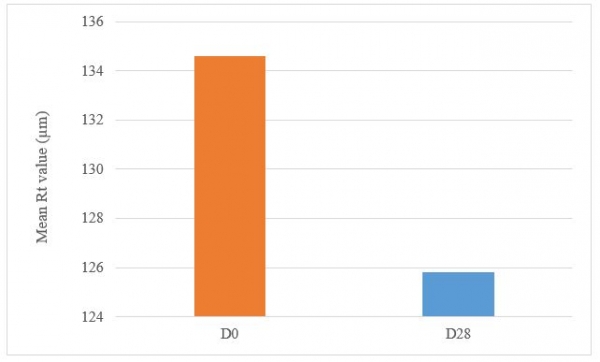
Decrease in the Rz and Rt parameters represents less wrinkled skin which shows the anti-wrinkle effect of the product.
Under these study conditions, the results show, after 28 days of twice daily use, Tagra CelluCap Resveratrol Lotion, induced significant anti-wrinkle effect evaluated by 3D Primos Lite System measurements, characterized by significant decrease in the average value of Rz and Rt parameters with observation of less wrinkle skin in majority of the tested subjects (figure 8 and 9).
Figure 7: Decrease in Wrinkle Relief from D0 to D28 following treatment twice daily with Tagra CelluCap Resveratrol Lotion (n; 22).
Figure 8: Decrease in the maximum relief amplitude Rt parameter following treatment twice daily with Tagra CelluCap Resveratrol Lotion (n; 22).
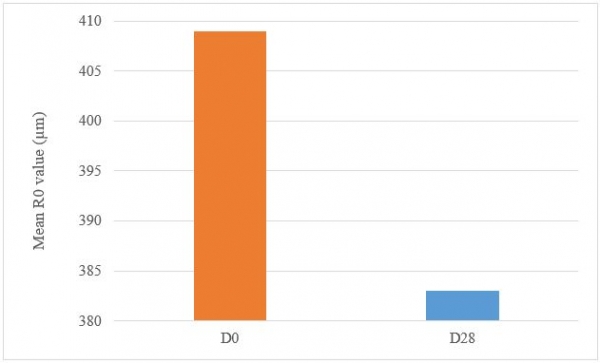
A significant decrease in R0 parameter presents a firming effect of the product. Under these study conditions, the results show, after 28 days of twice daily use, Tagra CelluCap Resveratrol Lotion, induced a significant firming effect evaluated by Cutometer System measurements, characterized by significant improvement in cutaneous firming parameter R0 with observation of firmer skin in majority of the tested subjects (figure 9).
Figure 9: Decrease in the firmness R0 parameter following treatment twice daily with Tagra CelluCap Resveratrol Lotion (n; 22).
Under these study conditions, the results show that the treatment with Tagra CelluCap Resveratrol Lotion was appreciated by majority of the subjects for its properties for its efficacy evaluated by subjective evaluation questionnaire. The results characterized by:
In a consumer study, after four weeks of use:
❖ 90% of participants agree that leaves the skin soft
❖ 81% of participants agree that leaves the skin moisturized
❖ 86% of participants agree that leaves the skin smooth
❖ 77% of participants agree that skin feels significantly firmer
❖ 86% agreed skin is healthier and fresh
❖ 86 % agreed that skin appears more luminous, and skin clarity is improved, leaving a healthy and even tone.
Conclusions
Resveratrol is a strong antioxidant ingredient with the potential to underpin significant skincare products. However, product developers face challenges and barriers when trying to incorporate resveratrol into innovative cosmetics. Resveratrol’s photo and thermal stability, along with its solubility and recrystallization issues, are major concerns.
Encapsulating resveratrol within the microcapsule structure protected and hermetically isolated the ingredient from the environment, reducing degradation following thermal and UV stimulation. The powder-form resveratrol microcapsules can be mixed into any formulation type at the cooling stage during product development, with no need to use solvents to dissolve the ingredient and no risk of recrystallization.
Another benefit of the resveratrol microcapsules is that the process is easy and does not need force or heat, unlike other drug delivery systems. We have successfully optimized and scaled up the formulation.
The microcapsules used Release on Demand technology. Rubbing the formulation into skin causes the polymeric shell to collapse, fracturing the microcapsules to release the active ingredient. As they breakdown, the microcapsules retain a portion of the payload and ingredient release continues over time.
Overall, the results obtained support the use of encapsulated resveratrol in topical cosmetic products. Microencapsulation stabilized resveratrol, ensuring the ongoing potency of the ingredient and its compatibility within formulations.
References
1. R. Pangeni, J. K. Sahni, J. Ali, S. Sharma, S. Baboota, Resveratrol: review on therapeutic potential and recent advances in drug delivery, Expert Opinion on Drug Delivery, 11(8) (2014) 1285-1298.
2. S. Bo, G. Ciccone, A. Castiglione, R. Gambino, F. De Michieli, P. Villois, M. Cassader, Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial, Current Medicinal Chemistry, 20(10) (2013) 1323– 1331.
3. L. Paulo, M. Oleastro, E. Gallardo, J. A. Queiroz, F. Domingues, Anti-Helicobacter pylori and urease inhibitory activities of resveratrol and red wine, Food Research International, 44(4) (2011) 964–969.
4. Baxter RA. Anti-aging properties of resveratrol: review and report of a potent new antioxidant skin care formulation. J Cosmet Dermatol. 2008 Mar;7(1):2-7.
5. Adhami VM, Afaq F, Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-kappaB in normal human keratinocytes by resveratrol. Neoplasia. 2003 Jan-Feb;5(1):74-82.
6. Chen ML, Li J, Xiao WR, et al. Protective effect of resveratrol against oxidative damage of UVA irradiated HaCaT cells. Jhong Nan Da Xue Xue Bao Yi Ban. 2006 Oct; 31(5):635–9.
7. Farris P, Yatskayer M, Chen N, Krol Y, Oresajo C. Evaluation of efficacy and tolerance of a nighttime topical antioxidant containing resveratrol, baicalin, and vitamin e for treatment of mild to moderately photodamaged skin. J Drugs Dermatol. 2014;13(12):1467-72.
8. Future Market Insights. Resveratrol Market to Grow at 8.1% CAGR by 2028 – Future Market Insights. GlobeNewswire News Room (2019). Available at: http://www.globenewswire.com/news-release/2019/03/29/1788521/0/en/Resveratrol-Market-to-Grow-at-8-1-CAGR-by-2028-Future-Market-Insights.html. (Accessed: 19th June 2019)
9. Š. Zupančič, Z. Lavrič, J. Kristl, Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature, European Journal of Pharmaceutics and Biopharmaceutics, 93 (2015) 196–204.
10. Q. Sun, J. Heilmann, B. König, Natural phenolic metabolites with anti-angiogenic properties–a review from the chemical point of view, The Beilstein Journal of Organic Chemistry,11(1) (2015) 249–264.
11. G. Davidov-Pardo, D. J. McClements, Resveratrol encapsulation: designing delivery systems to overcome solubility, stability and bioavailability issues, Trends in Food Science & Technology, 38(2) (2014) 88–103.
12. Y. O. Jeon, J. S. Lee, H. G. Lee, Improving solubility, stability, and cellular uptake of resveratrol by nanoencapsulation with chitosan and γ-poly (glutamic acid), Colloids and Surfaces. B: Biointerfaces, 147 (2016) 224–233.
13. C. Hu, Q. Wang, C. Ma, Q. Xia, Non-aqueous self-double-emulsifying drug delivery system: A new approach to enhance resveratrol solubility for effective transdermal delivery, Colloids and Surfaces. A: Physicochemical and Engineering Aspects, 489 (2016) 360–369.
14. TAGRA. Tagravit R article
15. TAGRA. Tagravit C article
16. Jelvehgari M, Montazam SH. Comparison of microencapsulation by emulsion-solvent extraction/evaporation technique using derivatives cellulose and acrylate-methacrylate copolymer as carriers. Jundishapur Journal of Natural Pharmaceutical Products.2012; 7:144-152.
17. Freitas S, Merkle HP, Gander B. Microencapsulation by solvent extraction/evaporation: reviewing the state of the art of microsphere preparation process technology. Journal of Controlled Release.2005; 102: 313-332.