Promotional Features
Seaweed science delves into skin microbiome
Natural, sustainably sourced ingredients supported by scientific evidence represent the skincare trifecta. Ticking all three key boxes - and more - high purity marine extracts are riding a global wave of momentum. Their credentials resonate not only with discerning consumers, but with new product developers searching for efficacious ingredients that are natural, easy to formulate and are not tested on animals.
Fucoidan, a bioactive polysaccharide found naturally in brown seaweeds, is a prime example of an innovative marine ingredient with clinically proven benefits for skincare applications. Fucoidan is the slimy film found on brown seaweeds. It serves to protect the plant from common external stresses such as UV radiation, marine pathogens and viruses. These same protective benefits have now been captured for the benefit of skin health. Topical fucoidan extracts developed specifically for cosmetic use have been commercially available for the past decade, with two high purity proprietary ingredients leading the charge.
Maritech Bright and Maritech Reverse herald from the global leaders in fucoidan science, Australian biotechnology company Marinova. The Maritech fucoidan brand, largely by virtue of its unique extraction process, is the world’s only high purity, certified organic fucoidan with global regulatory acceptance.
Above: Maritech organic fucoidan - the world’s only high purity, certified organic fucoidan with global regulatory acceptance.
Maritech Bright: A unique fucoidan-polyphenol complex for skincare
Maritech Bright is a natural, clinically validated skin brightening ingredient extracted from polyphenol rich Fucus vesiculosus seaweed, commonly known as bladderwrack. This COSMOS organic-certified skincare ingredient combines the potent bioactivity of fucoidan with the highly antioxidant and skin brightening properties of marine polyphenols.
Previous in vitro and human clinical testing has shown that this high purity fucoidan-polyphenol complex is safe, non-sensitising and highly effective at brightening skin. Studies have demonstrated reductions in pigment and age spots, increased brightness, decreased colour spot intensity and the inhibition of tyrosinase - the enzyme responsible for melanin synthesis.1.2
Research has also shown benefits in terms of healthy ageing. These benefits include smoothed skin and improved wrinkle depth, as well as the increased expression of the SIRT1 anti-ageing protein.1 Maritech Bright has demonstrated an ORAC 6.0 score more than double that of Vitamin C, and a superoxide scavenging ability more than 86 times higher than resveratrol and 38 times higher than curcumin. It has also been shown to protect against free radicals caused by UV exposure, pollution and environmental stresses.1
Above: Wild Fucus vesiculosus seaweed growing in the pristine coastal waters of Nova Scotia
Maritech Reverse: High purity fucoidan for skincare
Maritech Reverse is a high purity, certified organic fucoidan extract with clinically proven soothing, protecting and healthy ageing properties. This award-winning extract is derived from wild grown Undaria pinnatifida seaweed (commonly known as wakame or mekabu) harvested from the pristine waters of Tasmania and Patagonia. Maritech Reverse is COSMOS organic-certified and has undergone extensive in vitro and human clinical testing.1,3,4
Studies have shown that the potential benefits of Maritech Reverse include increased skin elasticity, reduce wrinkle depth, the inhibition of collagenase and elastase, and the increased expression of the SIRT1 anti-ageing protein. 1,3,4
Above: Wild Undaria pinnatifida seaweed growing in clean Tasmanian waters
Focus on: skin microbiome
Skin microbiome has been hailed as the wave of the future across the beauty industry. Ingredients that can assist in maintaining an optimal balance of bacteria on the skin are increasingly of interest to new product developers. Recent research into the effects of Maritech Bright and Maritech Reverse on skin microbiome suggests that both high purity fucoidan extracts have the potential to support a healthy dermal barrier and assist in the balancing of helpful and harmful bacteria.5
This latest study comes as high purity fucoidan is increasingly being utilised as an active ingredient in topical skincare and dermatological formulations. Fucoidan has been extensively researched and is particularly well known for its anti-inflammatory effects including limiting inflammation caused by allergic conditions.6
Balancing
Results of a recent in vitro study utilising a 3D model of atopic dermatitis, the most common form of eczema, suggested that Fucus vesiculosus and Undaria pinnatifida fucoidan extracts for dermatologic use, comparable to Maritech Bright and Maritech Reverse, may have a future role to play in the topical treatment of inflammatory skin conditions.5 The study showed that both fucoidan extracts beneficially altered the expression of genes typically associated with skin barrier function, wound healing processes and fluid accumulation.
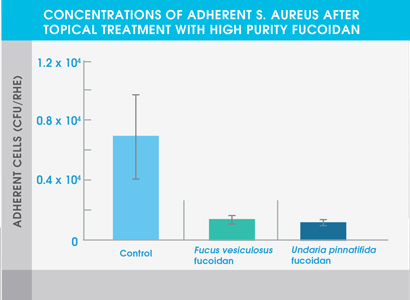
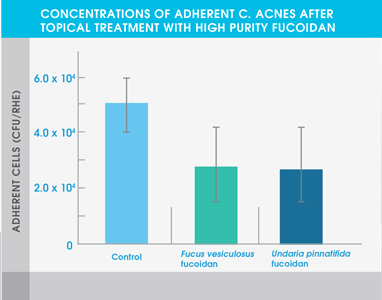
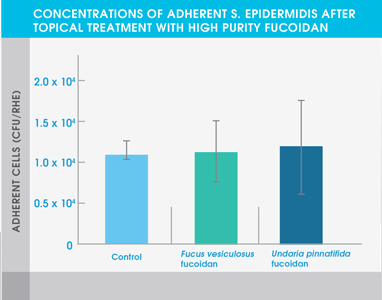
The study also showed that both high purity fucoidan extracts significantly inhibited the growth of bacteria typically abundant in the skin of eczema patients and acne patients – Staphylococcus aureus and Cutibacterium acnes respectively. Neither extract affected the adhesion of Staphylococcus epidermidis, a common bacterium found on healthy skin.
Figure 1. Concentrations of adherent S. aureus after topical treatment with high purity fucoidan
Figure 2. Concentrations of adherent C. acnes after topical treatment with high purity fucoidan
Figure 3. Concentrations of adherent S. epidermidis after topical treatment with high purity fucoidan
There is currently an unmet global need for effective, non-toxic treatments for debilitating inflammatory skin conditions such as eczema and acne.5 Atopic dermatitis currently affects up to 20% of children globally and up to 3% of adults.7 Commonly characterised by skin redness and intense itching, the effects can stretch far beyond appearance and negatively impact sleep habits, school and work performance, social confidence and emotional wellbeing. Having similar debilitating effects, acne is estimated to affect 9.4% of the global population, making it the eighth most prevalent disease in the world.8
These latest results suggest fucoidan may be a useful topical agent to assist in the management of such conditions, potentially offering relief to millions of sufferers.
Maritech extraction technology
Manufacturers of fucoidan have traditionally used solvents to precipitate fucoidan, degrading the natural compound and reducing bioactivity. Marinova has developed the unique proprietary Maritech extraction process, using advanced filtration technologies to separate and purify fucoidan without the use of harsh solvents.
Safety and formulation
All Maritech fucoidan extracts are certified organic and have global regulatory acceptance. Extensive clinical testing has confirmed that Maritech Bright and Maritech Reverse are safe for topical application. They are both free from preservatives, non-sensitising, non-irritating, non-allergenic and have never been tested on animals.
Human clinical and in vitro testing has confirmed the bioactivity of Maritech Bright and Maritech Reverse at inclusion rates ranging between 0.1-0.3% w/v. Maritech Bright and Maritech Reverse are both supplied as a dry powder. These ingredients are water soluble and ideal for inclusion in creams, gels, serums, masks and other common topical delivery systems. They can be utilized as the stand-alone active or easily incorporated with other ingredients.
Recommended storage conditions are in a cool dry place in well-sealed containers that are protected from light, heat and moisture. Both extracts are shelf-stable for five years.
Sustainability
All Maritech organic fucoidan extracts are underpinned by Marinova’s genuine commitment to sustainability. Marinova only utilizes wild seaweeds, a rapidly renewable marine resource, from the cleanest ocean waters and all harvesting operations are conducted in accordance with world’s best practice. All seaweeds used are harvested on an environmentally sustainable basis. Marinova does not source seaweeds that have been grown or farmed in those parts of the world prone to industrial or human contamination or where environmentally sustainable harvesting practices cannot be assured.
Marinova’s sustainability practices align with key Sustainable Development Goals (SDGs) identified by the United Nations. The company’s state-of-the-art manufacturing facility in Tasmania is powered solely by renewable energy and utilises unique ‘green chemistry’ principles to produce fucoidan compounds of unrivalled quality. Marinova has succeeded in diverting all seaweed residues away from unproductive landfill and into new, value-added organic products for the horticultural sector.
For further details visit www.marinova.com.au
References
1. Fitton, J.H. et al. Topical Benefits of Two Fucoidan-Rich Extracts from Marine Macroalgae. Cosmetics 2015.
2. Research performed by Brunswick Laboratories Inc USA/sponsored by Marinova Pty Ltd.
3. Research performed by Dermatest Pty Ltd Australia/sponsored by Marinova Pty Ltd Australia
4. Unpublished research.
5. Park, A.Y. et al.Modulation of Gene Expression in a Sterile Atopic Dermatitis Model and Inhibition of Staphylococcus aureus Adhesion by Fucoidan. Dermatopathology 2021.
6. Fitton, J.H. et al. Therapies from Fucoidan: An Update. Marine Drugs 2015.
7. Langan S.M. et al. Atopic dermatitis. The Lancet. 2020.
8. Tan, J. K. L. et al. A global perspective on the epidemiology of acne. British Journal of Dermatology. 2015.