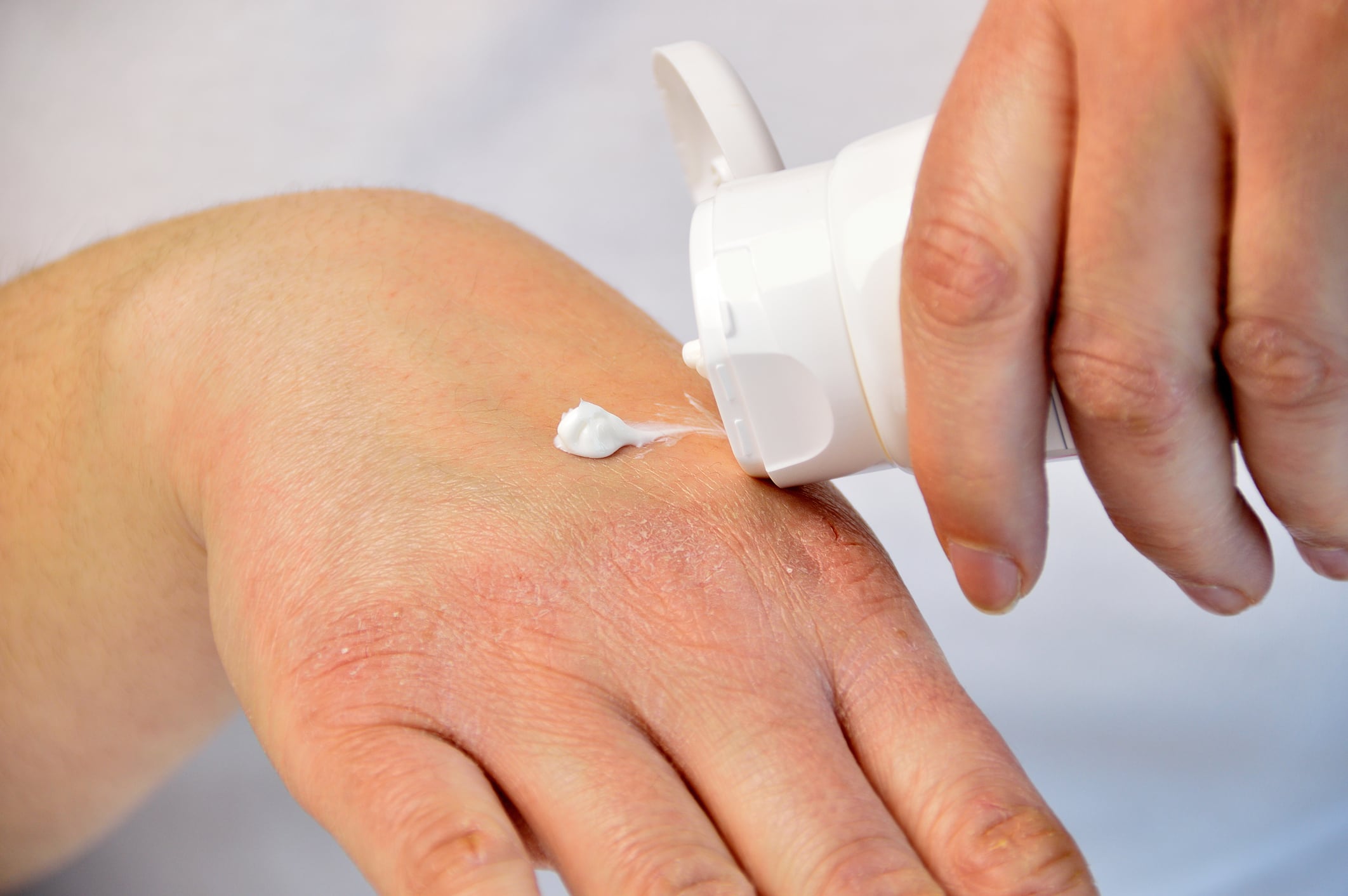
Atopic dermatitis, commonly called eczema, is a chronic inflammatory skin disease characterised by dry, itchy skin and rashes, and is most common in children. Numerous genetic and environmental factors contribute to eczema, and scientists are learning more about the role that the skin’s microbiome plays in this condition.
By conducting experiments in cell and animal models of eczema, NIAID (National Institute of Allergy and Infectious Diseases) scientists have previously found that a specific set of lipids (skin oils) produced by commensal skin bacterium Roseomonas mucosa can induce skin repair processes and promote turnover of skin tissue.
In 2019, NIAID researchers reported Interim results of a study involving 10 adults and five children (aged 9 to 14 years) given a topical application of the R. mucosa. The results indicated that the treatment was safe and associated with reduced eczema severity.
However, both mechanism of action and therapeutic impact of R. mucosa treatment in young children with AD under 7 years of age remained unexplored.
Since then, the trial enrolled an additional 15 children, for a total of 20 children with mild to severe eczema, aged 3-16 years.
In the recently reported study, children or their caregivers sprayed a solution of sugar water containing live R. mucosa onto areas of skin with eczema. For the first 15 children enrolled in the study, the dose of live R. mucosa was gradually increased each month. The last five children to enrol received the same dose throughout the four-month treatment period.
Researchers found that participants using the R. mucosa treatment displayed improvements in disease symptoms, epithelial barrier function, the amount of Staphylococcus aureus growing on the skin, the need for topical steroids, and quality of life for children and their families.
Seventeen of the 20 children experienced a greater than 50% improvement in eczema severity following treatment. Improvement occurred on all treated skin sites, including the inner elbows, inner knees, hands, trunk and neck. The scientists also observed increases in the skin’s barrier function—its ability to seal in moisture and keep out allergens.
Additionally, most children needed fewer corticosteroids to manage their eczema, experienced less itching, and reported a better quality of life following the therapy. These benefits persisted after treatment ended, and the therapeutic R. mucosa strains remained on the skin for up to eight months.
“A child suffering from eczema, which can be itchy, painful and distracting for the child, also is very difficult for the entire family,” said Anthony S. Fauci, M.D., director of NIH’s National Institute of Allergy and Infectious Diseases (NIAID), which led the study.
“These early-stage findings suggest that R. mucosa therapy may help relieve some children of both the burden of eczema symptoms and the need for daily treatment.”
“Most children in the study experienced substantial improvements in their skin and overall wellbeing following R. mucosa therapy. Encouragingly, the therapeutic bacteria stayed on the skin and continued to provide benefit after therapy stopped,” said NIAID’s Ian Myles, M.D., principal investigator of the trial."
This early study was designed to evaluate whether the safety and activity of R. mucosatreatment could justify progression to a large, randomised, placebo-controlled clinical trial.
“These results support a larger study to further assess the safety and effectiveness of this experimental treatment by comparing it with a placebo,” Myles adds.
Source: Science Translational Medicine
Myles. I. A., et al.
“Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair.”
DOI: 10.1126/scitranslmed.aaz8631 (2020).