Following various arguments and opinions by the Scientific Committee on Consumer Safety (SCCS), the list of preservatives is continuously changing.
Since the publication of EC Regulation n° 1223/2009, Annex V has been amended five times, notably to incorporate the prohibition of long-chain parabens and the restriction on the use of other preservatives such as triclosan and the Methylisothiazolinone/Methylchloroisothiazolinone (MI/MCI) mixture.
In 2015, methylisothiazolinone (MI) was the 5th most widely used preservative in Europe (1).
As the list of preservatives has been narrowed, rarely used preservatives were reasonably found more frequently in cosmetics. This result increasing the cases of allergies. This was the case for MI.
In 2016, two regulations have been introduced in order to take this phenomenon into account, resulting in additional constraints to the list of preservatives.
Towards the prohibition of MI in Europe?
So far, Methylisothiazolinone (MI) was authorized for use in all products at a concentration of 0.01%.
In 2016, two regulations have been introduced to prohibited MI in leave-on products and reduce its concentration to 15ppm (0.0015%) in rinse-off products.
These two regulations are at different stages:
- Adopted on July 23, 2016, the regulation EC n°2016/1198 prohibits the use of Methylisothiazolinone in leave-on products. Since 12 February 2017, only rinse-off cosmetic products shall be placed and made available on the Union market.
It remains, for the time being, authorized as a preservative in rinse-off products at a maximum concentration of 0.01% (100 ppm).
- The draft regulation aim of reducing its maximum concentration in rinse-off products at 0.0015% (15ppm). A public consultation was open to stakeholders until July 2016. The draft is now open for comments to WTO state members. This draft regulation is expected to be officially published in June 2017with two deadlines:
- from six months after the date of entry into force, only compliant cosmetics shall be placed on market
- a compulsory withdrawal within nine months following the date of publication
Manufacturers arise the question of the effectiveness of the MIT at this concentration.
The falling number of preservatives available for use in cosmetic products is one of the key challenges to the cosmetics sector, because it is crucial to maintain a diverse palette of preservatives to cover the multiple categories of products and hence protects efficiently consumers.
COSMED, with other stakeholders from industry and Member states, decided to tackle this issue within a specific working group established by the European Commission.
What happens worldwide?
Some countries like Canada, China, Korea, Turkey are in the footsteps of Europe.
Many countries are still following the former European Directive n°76/768/EEC. Not all of the recent amendments have been yet transposed into local regulations.
Hence, MI or MI/MCI mixture can currently be used indifferently in rinse-off and leave-on products. It is the case for example in MERCOSUR or gulf countries (Saudi Arabia, United Arab Emirates…).
At the opposite, a large majority of African countries or Chile do not currently impose any restriction on MI or MI/MCI mixture.
In USA, few cosmetic ingredients are regulated. Nethertheless, FDA recommends to manufacturer to follow CIR’S safety assessment. Thus, MI is safe at concentrations up to 100 ppm and safe for use in leave-on cosmetic products when formulated to be non-sensitizing which may be determined based on a quantitative risk assessment (QRA). For the MI/MCI mixture, CIR recommends to use it at maximal concentration of 15ppm in rinse-off products and 7.5 ppm in leave-on products.
An overview of the maximum concentrations permitted in key countries:
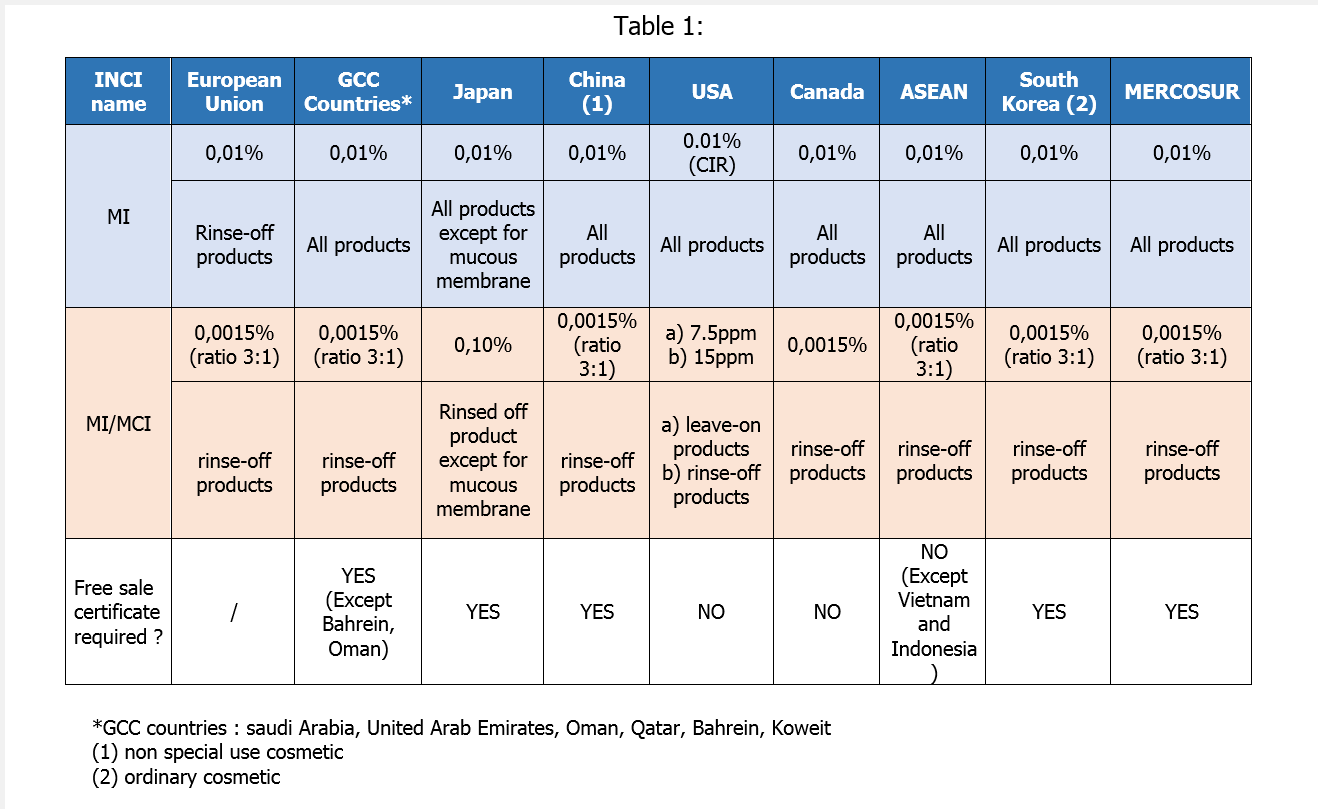
It is important to draw your attention that around sixty countries require a Free Sale Certificate (FSC). This therefore implies a conformity with EC Regulation n°1223/2009 for cosmetic product. In this case, the responsible person guaranties the validity of the FSC and by this the compliance to European Regulation at the time of shipment.
Since April 1, 2010 COSMED has been empowered to issue FSCs according to the specifications settled by the Ministry of Industry. Thus, COSMED FSCs are recognized in all the applicant countries requiring this document. For more information about FSC, visit our website.
