Regulation explained: part 2
Expert offers clarity on ‘Responsible Person’ requirement

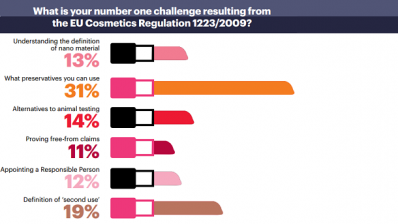
As part of the new rules, cosmetics companies must now nominate a single responsible person, based in the EU, whose remit is to ensure the compliance of each product with the rules set out in Regulation No 1223/2009.
“The sector, judging by the conversations we are having with customers, feels there’s a lack of clarity about this,” Gleghorn tells CosmeticsDesign-Europe.com.
“For instance, if you are importing cosmetics into Europe should the responsible person be based in the EU or be US-headquartered? The nominated individual has to be in the EU however, although this is not at all clear.”
Gleghorn says this is a huge responsibility, covering as it does manufacturing, testing on animals, consumer safety and customer communications.
Cosmetovigilance
The role also includes ‘Cosmetovigilance’, which is the continuous monitoring and recording of all undesirable effects in a health context that could be due to the use of cosmetic products.
“The single responsible person is furthermore duty-bound to maintain a ‘Product Information File’ readily accessible to the public authorities, and in a format they can understand,” explains Gleghorn.
To ensure product traceability, the nominated person therefore must identify the distributors of each product – information which must be kept for a period of three years following the date on which the batch of the cosmetic product was made available to the distributor.
Equivalent information must also be recorded for all other touch-points across the supply chain.
“To give an example, let’s say you have an adverse effect incident,” continues the Kallik boss.
“If anybody within the European community wants to get in touch about such a problem, then this responsible person is the contact they need to reach out to – and they may need to provide the Product Information File document if it is one of the competent authorities that is requesting information.”
Gleghorn concludes that there is still a lot to be agreed on what the so-called 'Responsible Person' is and how they are to be labelled on packs; but the change adds up to a special focus on one individual within the EU market to pass out information regarding the product.
It is thought with the new changes such as this, there will be clear benefits which will help achieve greater operational transparency, and Gleghorn believes the U.S. is likely to follow suit and move towards harmonisation with the EU.